New DNA-Based Imaging Technique Promises to Illuminate Cells and Tissues
Jan. 26, 2026
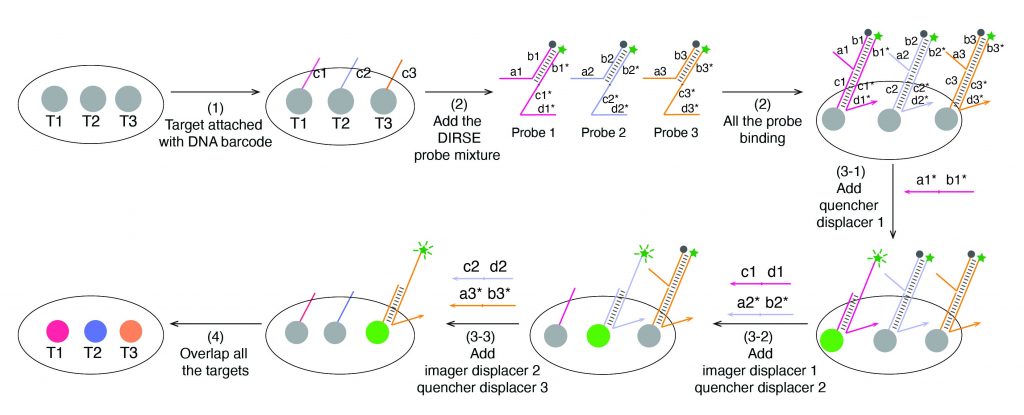
A team of researchers at UF chemistry has developed a transformative method to visualize the inner workings of cells with unprecedented detail. Traditional fluorescent imaging techniques, which use fluorophore color-coded markers to track molecules in cells, are typically limited to only a handful of targets at a time because fluorescent signals tend to overlap.
The new method, called DNA displacement reaction-based signal exchange (DIRSE), recently published in the Journal of the American Chemical Society, overcomes this barrier by using specially designed DNA probes that can be switched on and off by DNA strands. Each probe binds to its target and emits a fluorescent signal, which can then be removed by another DNA strand, freeing the channel for the next target. This process eliminates the slow, complicated washing steps required in conventional methods and works directly on standard microscopes.
Using this approach, the team achieved 25-plex RNA imaging in just 20 minutes in fixed cells, all within a single fluorescent channel. They also demonstrated 24-plex imaging in mouse retinal tissue, allowing them to distinguish different cell types in complex tissue environments.
Because DNA sequences can be designed in nearly unlimited combinations, the method could theoretically scale to visualize hundreds of targets simultaneously. This research, led by Yanju Chen and Ethan Xu at Hong Group, is in collaboration with Harvard Medical School.
This bioanalytical method offers a faster, simpler, and more accessible way to study cellular organization and function, with potential applications in biotechnology, medicine, and diagnostics. By unlocking high-dimensional molecular imaging without complex instruments, DIRSE could transform how scientists explore the inner life of cells.
Read the paper
Learn more about the Hong lab
