Electrochemistry Unlocks Low-Temperature Depolymerization
Jan. 19, 2026
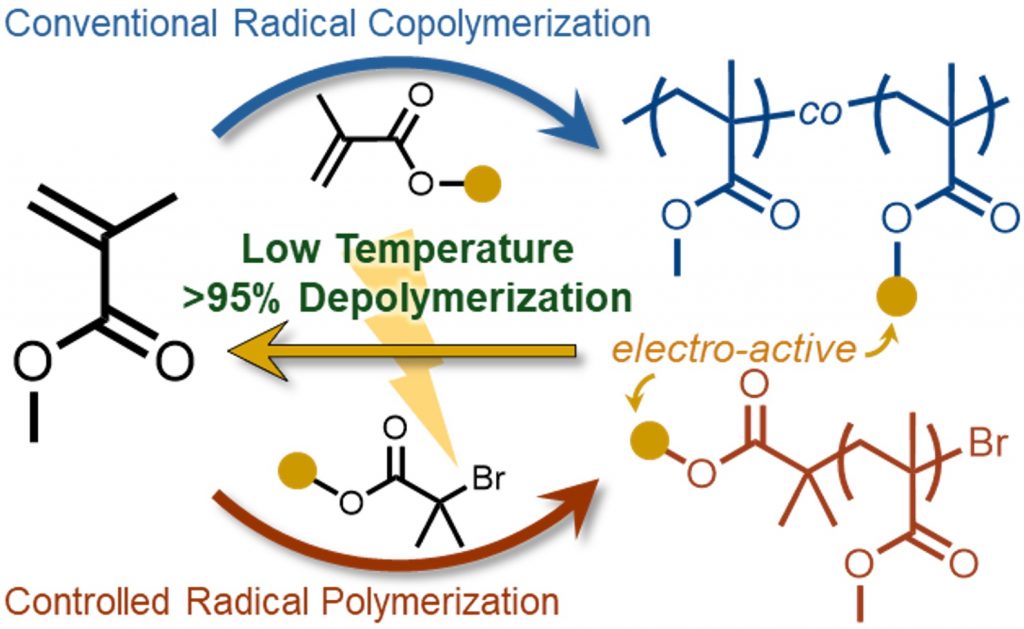
Efficient chemical recycling of plastics with all-carbon backbones remains one of the central challenges in polymer sustainability. Many widely used materials, including poly(methyl methacrylate) (PMMA), currently require extreme conditions to break down, often exceeding 400 °C. In a new collaborative study from the Butler Polymer Research Laboratory, a fundamentally new strategy has been developed that dramatically lowers this barrier by decoupling radical generation from thermal activation.
As recently reported in the Journal of the American Chemical Society, the team developed the first example of electrochemically initiated depolymerization of PMMA. By using an electrochemical stimulus to generate polymer-centered radicals, followed by thermal depropagation, PMMA can be efficiently unzipped back to its monomer at temperatures as low as 105 °C. This orthogonal, stimulus-separated approach represents a conceptual shift in how vinyl polymer depolymerization can be achieved.
The research was led by graduate student Graham Gilchrist, who is coadvised by Professors Brent Sumerlin and Austin Evans. The project grew out of a close collaboration between the two research groups, blending expertise in polymer chemistry and electrochemistry to tackle a long-standing limitation in plastic recycling. Gilchrist played a central role in designing the molecular systems, conducting mechanistic studies, and developing the experimental platform that made the advance possible.
Beyond establishing proof of concept, the study provides detailed insight into how polymer structure influences depolymerization efficiency. The researchers show that radical initiation at chain ends is effective for lower molecular weight polymers but becomes less efficient as chain length increases. In contrast, introducing electrochemically active pendent groups enables highly efficient depolymerization of high-molecular-weight PMMA, with greater than 95 percent monomer recovery at modest incorporation levels.
Building on this understanding, the team designed a custom electro-distillation apparatus that allows depolymerization and monomer purification to occur simultaneously. As PMMA is electrochemically activated and depolymerized, methyl methacrylate is distilled off in real time, offering a potential pathway toward closed-loop recycling where recovered monomer can be directly repolymerized.
Together, these findings establish electrochemistry as a versatile and previously unexplored stimulus for vinyl polymer depolymerization. The work opens new directions for designing recyclable plastics and highlights the impact that student-driven, interdisciplinary collaboration can have in addressing major sustainability challenges.
Read the paper
Learn more about the Sumerlin lab
Learn more about the Evans lab
